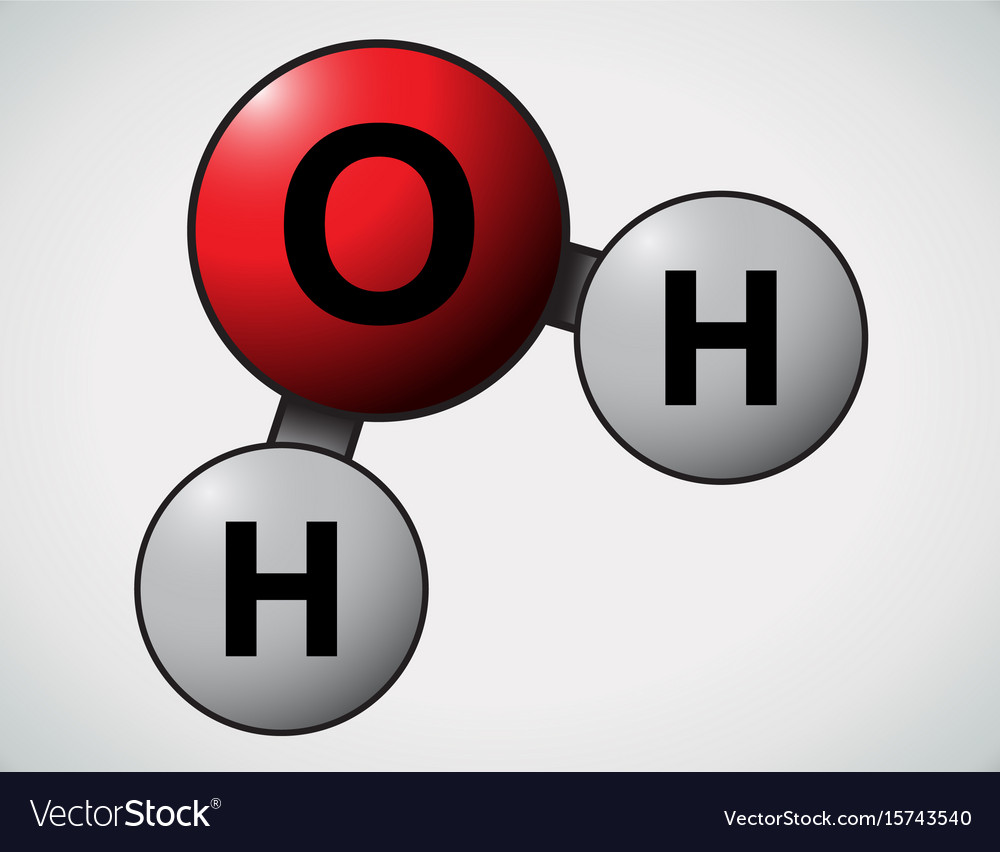
How Many Carbon Atoms In Water .to achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ).
from www.ouestny.com
Carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). A single oxygen atom contains six electrons in its outer.
How many atoms is in water?
How Many Carbon Atoms In Water Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ).to achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A single oxygen atom contains six electrons in its outer.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Many Carbon Atoms In Water a water molecule is made up of two hydrogen atoms and one oxygen atom. A single oxygen atom contains six electrons in its outer. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent.to achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and. How Many Carbon Atoms In Water.
From iperiodictable.com
Electron Configuration of Carbon Periodic Table Element How Many Carbon Atoms In Waterwater is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A single oxygen atom contains six electrons in its outer. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent.carbon dioxide. How Many Carbon Atoms In Water.
From www.shutterstock.com
Glossy Vector Illustration Showing The Composition Of Three Different How Many Carbon Atoms In Water Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ).water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent.carbon dioxide makes up only a small part of the. How Many Carbon Atoms In Water.
From stock.adobe.com
illustration of chemistry, The diamond structure each carbon atom forms How Many Carbon Atoms In Water Plants use carbon dioxide in the. A single oxygen atom contains six electrons in its outer.to achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent. Because of the higher electronegativity. How Many Carbon Atoms In Water.
From www.ces.fau.edu
Climate Science Investigations South Florida Causes of Climate Change How Many Carbon Atoms In Watercarbon dioxide makes up only a small part of the atmosphere (about 300 parts per million), but it is a crucial gas.a common example is the dissolving of carbon dioxide in water, resulting in carbonated water. A single oxygen atom contains six electrons in its outer. Because of the higher electronegativity of the oxygen atom, the bonds. How Many Carbon Atoms In Water.
From www.youtube.com
Number of Atoms in CO2 (Carbon dioxide) YouTube How Many Carbon Atoms In Waterwater is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent. A single oxygen atom contains six electrons in its outer. Plants use carbon dioxide in the. Water is a simple molecule consisting of one oxygen atom bonded to two. How Many Carbon Atoms In Water.
From www.eupschools.org
REMC 22 / Figure App How Many Carbon Atoms In Watera common example is the dissolving of carbon dioxide in water, resulting in carbonated water. a water molecule is made up of two hydrogen atoms and one oxygen atom. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent.water is a simple molecule consisting of one oxygen atom bonded to two different. How Many Carbon Atoms In Water.
From www.slideserve.com
PPT The Number of Atoms in a Formula PowerPoint Presentation, free How Many Carbon Atoms In Water Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.carbon dioxide makes up only a small part of the atmosphere (about 300 parts per million), but it is a crucial gas. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent. Plants use carbon dioxide in the. A. How Many Carbon Atoms In Water.
From info.teledyne-hi.com
Carbon Dioxide (CO2) How Many Carbon Atoms In Water Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.a common example is the dissolving of carbon dioxide in water, resulting in carbonated water. A single oxygen atom contains six electrons in its outer. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent.to achieve stability,. How Many Carbon Atoms In Water.
From teacher-history.ru
Что такое ковалентная связь в химии? teacherhistory.ru How Many Carbon Atoms In Water Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent.water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.a common example is the dissolving of carbon dioxide in water, resulting in carbonated water. Carbon dioxide is weakly soluble in water, therefore it separates into a gas. How Many Carbon Atoms In Water.
From www.nagwa.com
Question Video Determining the Number of Carbon Atoms in an Alkene How Many Carbon Atoms In Waterwater is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. a water molecule is made up of two hydrogen atoms and one oxygen atom. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent. How Many Carbon Atoms In Water.
From brainly.com
Explain how the models you developed show that when methane combines How Many Carbon Atoms In Water Carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent. Because of the higher electronegativity of the oxygen atom, the bonds are. How Many Carbon Atoms In Water.
From www.ces.fau.edu
Climate Science Investigations South Florida Causes of Climate Change How Many Carbon Atoms In Water Carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. a water molecule is made up of two hydrogen atoms and one oxygen atom. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent.a common example is the dissolving of carbon dioxide in water, resulting. How Many Carbon Atoms In Water.
From www.coursehero.com
Collision Theory Chemistry Course Hero How Many Carbon Atoms In Waterto achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule. a water molecule is made up of two hydrogen atoms and one oxygen atom. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Water is. How Many Carbon Atoms In Water.
From www.coursehero.com
[Solved] . Identify all carbon atoms, missing hydrogen atoms, and lone How Many Carbon Atoms In Water Plants use carbon dioxide in the. Carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. A single oxygen atom contains six electrons in its outer.water is a simple molecule consisting of one oxygen. How Many Carbon Atoms In Water.
From www.worldatlas.com
What Is The Carbon Cycle? How Many Carbon Atoms In Water Carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released.carbon dioxide makes up only a small part of the atmosphere (about 300 parts per million), but it is a crucial gas. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Web. How Many Carbon Atoms In Water.
From www.shutterstock.com
Carbon Dioxide Co2 Atom Model Illustration Stockvektor (royaltyfri How Many Carbon Atoms In Waterwater is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms.to achieve stability, carbon must find four more electrons to fill its outer shell, giving a total of eight and satisfying the octet rule.carbon dioxide makes up only a small part of the atmosphere (about 300 parts per million), but. How Many Carbon Atoms In Water.
From scienceatyourdoorstep.com
Types of Atoms Science at Your Doorstep How Many Carbon Atoms In Watera common example is the dissolving of carbon dioxide in water, resulting in carbonated water.carbon dioxide makes up only a small part of the atmosphere (about 300 parts per million), but it is a crucial gas. A single oxygen atom contains six electrons in its outer. a water molecule is made up of two hydrogen atoms. How Many Carbon Atoms In Water.